What is medical grade printing ink?
Medical grade printing ink in this sense, refers to pad printing ink specifically made for PPE, apparel or medical devices. Ink that is recognized as ‘medical grade’ must go through numerous testing procedures to ensure that it is fit for human contact. For example, face masks must follow and pass several testing procedures in accordance with the American Society for Testing Materials (ASTM) in the U.S. or European Standards (EN) in Europe. For medical devices, the ink must go through numerous rounds of testing to ensure it meets the specifications put forward by the U.S. Pharmacopeial Convention (USP). This organization’s main purpose is to create standards for medications, healthcare technologies and more so that human tissue to part contact is compatible and without harm. The USP has six different designations for plastics. Class VI is the most stringent.
What type of testing is done to ensure that this ink is safe for human contact?
Depending on your product and intended use, there are quite a few testing methods that medical products can adhere to:
Medical Apparel such as Scrubs and Hospital Gowns (non-surgical- Level 1)
For all ink that comes into regular contact with skin and is on fabric, specific testing such as Oeko-Tex and NAMSA are standard in the U.S. while REACH is standard in Europe.
Oeko-Tex
Oeko-Tex tests the final product to ensure that it does not contain any harmful substances. This strict testing method tests every fiber and finishing of the product. For scrubs and hospital gowns that cloth those who will have long-term contact with the ink, it is imperative to ensure there is no adverse reaction between the skin and the final product.
NAMSA
Like Oeko-Tex, NAMSA studies the long-term effect of direct or indirect skin contact with the printed product. This refers to contact with the user and the patient. If there is no irritation when in contact with skin, it is considered safe.
REACH
REACH or Registration, Evaluation, Authorization and Restriction of Chemicals takes account the medical pad printing ink itself. It tests for harmful chemicals to humans as well as to the environment. This testing is required for all products sold and distributed in Europe.
Personal Protective Equipment such as Face Masks and Medical Gowns (Surgical)
Face masks, medical gowns and other types of PPE are worn to prevent the spread of fluids, particles and other harmful pathogens that get people sick. While the FDA regulates PPE for medical use, general purpose or PPE for industrial use is not regulated by this organization. For the purpose of this article, we will focus on regulated, medical PPE.
PPE for medical use must be labeled appropriately to comply with FDA standards as well as safely alert the user as to how the product should be used. For example, medical gowns range from Level 1 to Level 4 with Level 4 being the ‘high-risk’ protective barrier. Since a Level 2 medical gown does not protect the user from infectious diseases, it is important to alert the user to not use it for such patients or contact. A mislabel or a label that wears easily, will not be able to appropriately alert the user and can cause an accident. With labeling PPE, there is a three-fold issue here. First, it is ensuring the ink does not irritate the patient nor the user. Second, it is ensuring the label cannot easily wear-off as to not properly alert its specific use. Third, it is ensuring the process of labeling does not alter the composition of the product.
As with many other products, the ASTM has developed an umbrella document outlining what physical properties such gowns should have while the ANSI and the AAMI outline more of the protective properties. Additionally, NOISH has outlined particular face masks and respirators for healthcare workers to keep them as safe as possible.
Since PPE has direct or indirect contact with humans, it is important that the ink used to label it complies with its corresponding medical standards.

Medical Devices and Similar Products
To pass Class VI certification, the ink must go through the following testing procedures:
- Acute Systemic Toxicity Test
- Intracutaneous Test
- Implantation Test
Combined, these testing methods will determine if the medical grade printing ink has a low level of toxicity. Once this passes, the next step is to subject the product to a series of temperature assessment tests. This series of tests does not verify the toxicity of permanently or semi-permanently products implanted into patients. For this, ISO-10993 testing may be required. ISO-10993 is a more rigorous testing procedure and actually looks at the interaction between the human body and the product for an extended period of time.
Take a deeper dive into the medical specifications of Class VI here: https://www.fostercomp.com/stewardship/usp-class-vi/
In addition to the toxicity verification, companies should keep in mind that many medical products use harsh chemicals to clean these devices after use. Rubbing alcohol, for example, is used to clean medical products after each use.

Why is pad printing the preferred method of medical printing?
Pad printing is considered a preferred method due to its flexibility, ability to easily label yet not alter the product’s intended use and its ability to pass the strictest safety testing.
Flexibility/Versatility
Companies are able to switch over from printing hard goods to apparel with a switch of the consumables. In just one day, a company can print all goods mentioned in this article without having to wait hours in between. Simply switching up the artwork, ink and pad can take you from printing on medical devices to printing labels on scrubs.
In addition to the ability easily switch over to different applications, the sheer number of substrates pad printing ink can stick to is astronomical. The key is to make sure the supplier you are working with is educated in different substrates and can properly guide you.
Learn more about Inkcups’ line of pad printing inks for different substrates.
Non-Product Altering Method
When it comes to labeling face masks, in particular, it is key to have a method to properly label the product with altering its use. For example, using a heat press to adhere a label onto the mask will disable the filtering and breathing properties of the mask. Additionally, some methods use chemicals to pre-treat the product which are harmful to breathe in.
Certified Ink
While not all pad printing inks are made the same, pad printing ink that is certified medical grade or shows no irritation on contact with skin is key for this particular industry. Be sure to ask your pad printing ink supplier to see the necessary documentation to ensure the piece does not poorly interact with human contact.
What inks does Inkcups have to offer the medical industry?
Inkcups has an array of inks that satisfy the needs of the medical industry:
Our MB Series has gone through the appropriate testing to be label medical grade (Class VI). It is the perfect pad printing ink for medical items such as syringes, catheter tubes, medical devices and more.
Like the MB Series, Inkcups’ J3 Series is also Class VI certified. The difference lies in what substrates each can adhere to.
The SB Series is primarily used for apparel such as scrubs, hospital gowns and numerous other fabrics. As our go-to apparel ink, the SB Series has been thoroughly tested and has passed the most strict compliance testing in terms of safety.
For our full list of compliance documentation for these inks, visit: Our Compliance Section
Inkcups is the leading manufacturer and supplier of industrial printing equipment including pad printing machines, UV printers, tagless t-shirt printing equipment and laser plate-makers. To complete the solution, Inkcups also is a supplier of UV inkjet ink, inkjet primers, pad printing ink, pad printing solvents, pad printing plates, pad printing pads and more. With offices on practically every continent, Inkcups is able to provide solutions and support across the globe.
For more information on medical product pad printing, please contact us today.
 Tagless Printers
Tagless Printers Cylindrical Inkjet Printers
Cylindrical Inkjet Printers UV Flatbed Printers
UV Flatbed Printers Pad Printing Machines
Pad Printing Machines Pretreatment Systems
Pretreatment Systems Inkjet Printing Auxiliary
Inkjet Printing Auxiliary Laser Plate-Makers
Laser Plate-Makers Inkjet Printing Supplies
Inkjet Printing Supplies Pad Printing Supplies
Pad Printing Supplies Tagless Supplies (tagless.inkcups.com)
Tagless Supplies (tagless.inkcups.com)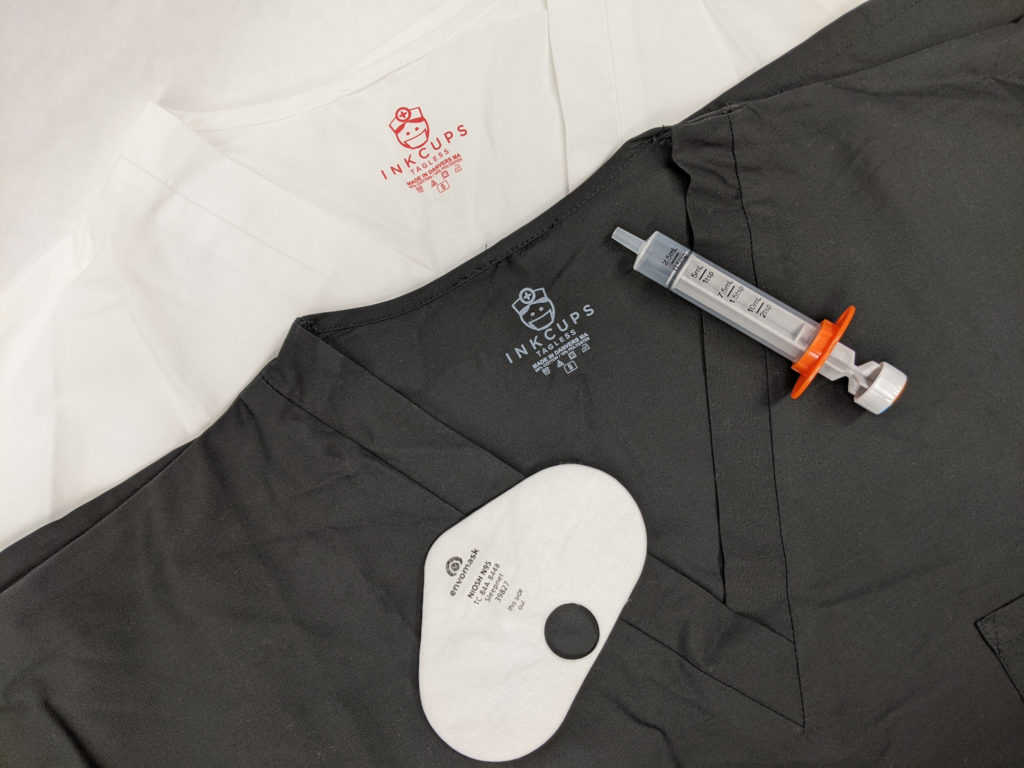



Add Your Comment